EDEM3-CDG: Austin, Savannah and Lily’s story
“When Rachel and Rob brought their 7-month-old baby boy to Children’s Hospital of Philadelphia (CHOP) for feeding issues and difficulty gaining weight, they had no idea it would launch a diagnostic odyssey that would take years to identify, wind up involving all three of their children and lead to the discovery of a gene not previously associated with a human genetic disease.
Today, the family is among just seven in the world to be diagnosed with ER Degradation Enhancing Alpha-Mannosidase Like Protein 3 congenital disorder of glycosylation (EDEM3-CDG), a newly recognized genetic condition.”
Read more about one family’s journey to CDG diagnosis here
New Patient Education Resource on GPI Disorders
The newest FCDGC patient education resource is now available!!
The Mayo Clinic and Frontiers in CDG Consortium developed a new patient education booklet on GPI Disorders: “GPI Anchor Disorders: A Subtype of Congenital Disorders of Glycosylation (CDG)”.
Access the resource on our website here!
2022 Rare Disease Day Symposium & CDG/NGLY1 Family Conference
REGISTRATION IS OPEN for the 2022 Sanford Burnham Prebys Rare Disease Day Symposium & CDG/NGLY1 Family Conference!
This joint scientific and family symposium will be held on February 25-27th at the Dana on Mission Bay in San Diego, CA.
The objective of this conference is to combine patient-centered science and real-world strategies for patients and families living with a Congenital Disorder of Glycosylation in a supportive and friendly environment. Join 150+ medical professionals and families in sharing their knowledge and experiences, and discuss common issues of patients living with CDG. The 2022 event is co-organized by Sanford Burnham Prebys and CDG CARE.
The scientific session will be held on February 25-26, and the family session on February 27, 2022.
To visit the event page and view the preliminary agenda, venue details and Buy Your Tickets CLICK HERE
Don’t miss out on one of the biggest CDG conferences of the year!
Updated FCDGC Guidance on COVID-19 Vaccine Ages 5+
The FCDGC updated guidance for CDG & NGLY1 patients 5+ years old and families receiving the COVID-19 vaccine is now available!
CDG families and community members can access the recommendation here
Treatment Options in Congenital Disorders of Glycosylation
“Despite advances in the identification and diagnosis of congenital disorders of glycosylation (CDG), treatment options remain limited and are often constrained to symptomatic management of disease manifestations. However, recent years have seen significant advances in treatment and novel therapies aimed both at the causative defect and secondary disease manifestations have been transferred from bench to bedside. In this review, [the authors] aim to give a detailed overview of the available therapies and rising concepts to treat these ultra-rare diseases.”
Read the review article here
Journal Reference:
Park & Marquardt. (2021) Treatment Options in Congenital Disorders of Glycosylation. Frontiers In Genetics. 10.3389/fgene.2021.735348
Reversibility of motor dysfunction in the rat model of NGLY1 deficiency
The first person with NGLY1 deficiency (NGLY1-CDDG) was identified in 2012. Since then, more than 60 new patients have been diagnosed worldwide. NGLY1 deficiency is caused by mutations in NGLY1 gene, resulting in a broad spectrum of clinical features including developmental delay, seizure, intellectual disability, movement disorder and a lack or absence of tears. It is not yet understood how mutations in the NGLY1 gene leads to the various symptoms of NGLY1 deficiency and no effective therapy is currently available.
A new study published by Dr. Tadashi Suzuki’s research team presents exciting results that may pave the way for developing gene therapies for NGLY1 deficiency. Previously, Dr. Suzuki’s team developed a rat model of NGLY1 deficiency and showed that the rats display similar symptoms to human NGLY1 deficiency patients (Read the Asahina et. al 2020 study here). In their recent study, Asahina and colleagues showed that administering an AAV9-based human NGLY1 gene to the central nervous system (CNS) of the mice reversed their movement disorder and increased the activity of the NGLY1 enzyme in the brain.
This study demonstrates that the rat model is useful for to evaluate potential therapies for NGLY1 deficiency and highlights the potential of gene therapy-based treatment.
Journal Reference:
Asahina et al. (2021) Reversibility of motor dysfunction in the rat model of NGLY1 deficiency. Molecular Brain. 10.1186/s13041-021-00806-6
New Informational Resource for NGLY1-CDDG!
A new informational resource is now available for the NGLY1 community on the NORD website!
Check it out here to learn more about the signs & symptoms of NGLY1 Deficiency, diagnosis, therapies and more!
New Genetic Marker Identified for Congenital Disorders of Glycosylation
A new genetic cause of Congenital Disorders of Glycosylation (CDG) has been identified, according to a recent study in The American Journal of Human Genetics. This means a blood sample can now confirm diagnosis of this form of CDG, notes Andrew C. Edmondson, MD, PhD, an attending physician in Human Genetics and Metabolism at Children’s Hospital of Philadelphia and co-author on the study. Edmondson is one of the founding members of Frontiers in Congenital Disorders of Glycosylation (FCDGC).
People born with CDG can experience a range of neurological symptoms, developmental issues, growth struggles, and organ problems. Due to the fact that CDG represent a variety of rare disorders with such a range of presentations, children with CDG are often misdiagnosed.
Using a combination of exome sequencing and gene matching, researchers identified 12 individuals from seven families who present with CDG with a variant in EDEM3, a gene which encodes a protein responsible for recognizing misfolded glycoproteins in the endoplasmic reticulum (ER). The presence of misfolded ER proteins is involved in several CDGs, so tracing this system was important.
Individuals in the study affected by CDG showed either homozygous or compound heterozygous variants in the EDEM3 gene. Unaffected parents were all heterozygous carriers. People from two families carried a common, identical region of homozygosity (with no records of inter-familial consanguinity); both families were of Portuguese Romani origin. A control group of 96 healthy control subjects in the Portuguese Romani population were also screened for this variant, with one heterogeneous allele detected. It’s possible links to this population may represent a founder effect.
The CDG patients in the study presented with developmental delay with intellectual delay and speech delay in some cases. Half showed hypotonia and many presented with dysmorphic facial features.
By comparing ratios of glycosylation abnormalities across affected CDG patients, researchers found a global impact on N-glycosylation in EDEM3 deficiency and suggest among EDEM3-CDG-affected people, abnormal N-glycan profiling patterns provide additional diagnostic biomarkers.
As Edmondson notes, EDEM3 knock out mice “had similar biochemical abnormalities in blood and brain as were seen in the human sample.” Now that the gene has been identified, future research is likely to focus upon how loss of EDEM3 activity leads to disease and this set of symptoms that are so often misunderstood or misdiagnosed.
Source:
Rare Diseases Clinical Research Network
Journal Reference:
Polla, D.L., et al. (2021) Bi-allelic variants in the ER quality-control mannosidase gene EDEM3 cause a congenital disorder of glycosylation. American Journal of Human Genetics. 10.1016/j.ajhg.2021.05.010
Study shows the mechanism how loss of de-N-glycosylation enzyme causes ill effect
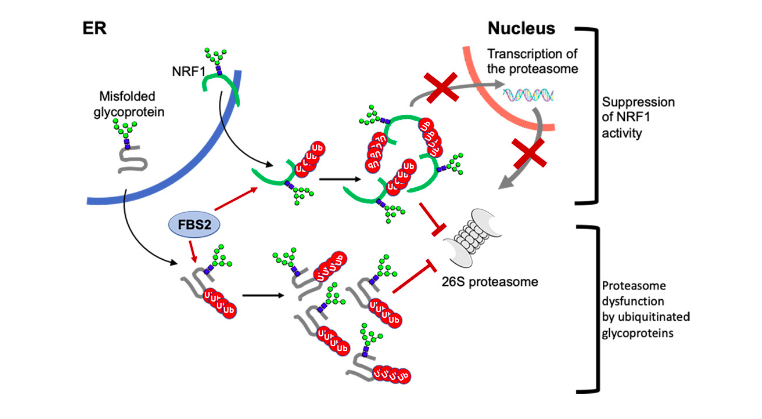
Image: A schematic model of the mechanism of proteasome dysfunction by FBS2 in NGLY1 deficiency. FBS2 induces cellular toxicity in the absence of NGLY by two steps. First, FBS2 ubiquitinates misfolded glycoprotein substrates. Ubiquitinated glycoproteins recruited to the proteasome can strongly inhibit the proteasome activity, because of the presence of N-glycans. Accumulation of ubiquitinated glycoproteins results in the inhibition of the proteasome. Cells sense the inhibition of the proteasome, they try to induce transcriptional activation of proteasome subunits by active NRF1. But FBS2 suppresses by inhibiting nuclear import of NRF1. Moreover, induced NRF1 also causes proteasome dysfunction as the ubiquitinated glycoprotein substrates.
Peptide: N-glycanase (NGLY1) is an evolutionarily conserved enzyme for removing N-linked glycans (N-glycans) from glycoproteins and is involved in proteostasis of N-glycoproteins in the cytosol. In 2012, a rare genetic disorder called NGLY1 deficiency was discovered by an exome analysis. Symptoms in patients with NGLY1 deficiency include global developmental delay, hypotonia, hypo/alacrima, movement disorder, scoliosis, abnormal liver, brain functions and peripheral neuropathy. Unfortunately, no therapeutic treatment is currently available to this devastating disease, because the exact mechanism of harm is not yet understood. So far, Dr Suzuki’s team in RIKEN and T-CiRA have developed various animal models for NGLY1 deficiency and reported that NGLY1-knockout in B6 mice is embryonically lethal.
In the cytosol, there are other proteins involved in processing of N-glycoproteins. One such group of proteins is glycoprotein-specific ubiquitin ligase subunits, FBS (F-box proteins recognizing sugar chains), which ubiquitinates unfolded glycoproteins in the cytosol. Ubiquitination of proteins serves as signals for proteolysis by the proteasome, a proteolytic machinery essential for cellular proteostasis and health. The researchers found that deletion of gene encoding FBS2, one of FBS proteins, restored the lethality of NGLY1-KO B6 mice and the FBS2;NGLY1 double-KO mice grew without apparent abnormalities. This finding reveals that the activity of FBS2 could cause the lethality in the embryos of NGLY1-KO mice.
The detrimental effects of FBS2 in the absence of NGLY1 were also observed in culture cells. The researchers noticed that FBS2 overexpression in NGLY1-KO cell lines suppressed cell growth and eventually induced cell death. FBS2 ubiquitinated glycoproteins that failed to remove N-glycans in the absence of NGLY1, and the ubiquitinated glycoproteins were not degraded and accumulated in cells. Ubiquitinated proteins are normally degraded by the proteasome, but ubiquitinated glycoproteins induced proteasome inhibition. The prolonged proteasome inhibition leads to cell death. In order to maintain proteasome function, cells induce the synthesis of new proteasomes by the transcriptional factor NRF1 upon proteasome dysfunction. Interestingly, NRF1 is a glycoprotein that resides in the endoplasmic reticulum and degraded by the proteasome under normal conditions. When proteasome activity is suppressed, NRF1 is stabilized and translocated into the nucleus where it activates transcription of the proteasome. However, in the absence of NGLY1, FBS2 ubiquitinated NRF1 and suppressed its transcriptional activity of the proteasome. Thus, FBS2 inhibited proteasome activity and also injured the recovery function of the proteasome in loss of NGLY1. The presence of ubiquitinated NRF1 was also observed in NGLY1-KO mouse embryos.
This work paves the way to development of therapeutics for this intractable disease.
Source:
Yoshida, Y., et al. (2021) Loss of peptide:N-glycanase causes proteasome dysfunction mediated by a sugar-recognizing ubiquitin ligase. Proceedings of National Academy of Sciences. doi.org/10.1073/pnas.2102902118